¿Cuál es más polar HI HCl HBr HF?
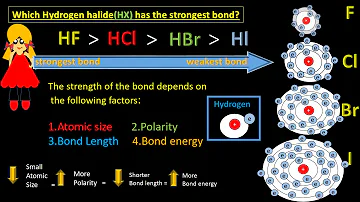
The order of polarity is as follows: HF>HCl>HBr>HI.
Is HCl and HBr are polar?
The following hydrogen halides (HF,HCl,HBr and HI) are all polar molecules.
Which is more polar HCl or HF?
HF is more polar than HI or HCl. HF forms stronger bond than HI. The extent of the ionic character of a covalent bond depends on polarity of molecule which in turn is proportional to the difference of electronegativity b/w the bonded atoms.
Which is more polar than HI or HF?
(B) The H-F bond is more polar than the H-I bond because fluorine is more electronegative than iodine.
Which is a more polar molecule HF or HBr?
which of the following hydrogen halides has the most polar molecule and why? HI,HBr,HCl,HF. Since fluorine is the most electronegative element and H is the most electropositive element, HF is the most polar.
Is HCl or HI more polar?
HCl is more polar than HI as the electronegativity difference between hydrogen and chlorine is greater when compared to the electronegativity difference between hydrogen and iodine.
Is HCl polar or nonpolar or polar?
polar covalent compound
HCl is polar covalent compound as the chloride ion is more electronegative than hydrogen ion. So chloride ion carries partial negative character while hydrogen carries partial positive character. Covalent character is shown by HCl as the atoms of hydrogen and chlorine share their electrons with each other.
Which is more polar HI or HBr?
The order of polarity is as follows: HF>HCl>HBr>HI.
Which is more polar HCl or HI?
HCl is more polar than HI as the electronegativity difference between hydrogen and chlorine is greater when compared to the electronegativity difference between hydrogen and iodine.
Why HCl is more polar than HBr?
Most people say that it is because the 3p orbital of chlorine when overlapping with the s orbital of hydrogen covers more area than when 4p orbital of bromine overlaps with the s orbital of hydrogen which makes bond between H and Cl more stronger than the bond between H and Br.
What is the polarity of HCl?
There and that the chlorine has the electrons. Much longer than the hydrogen. We can also visualize that as cloud of electrons.
Is HCl polar or polar?
polar covalent compound
HCl is polar covalent compound as the chloride ion is more electronegative than hydrogen ion. So chloride ion carries partial negative character while hydrogen carries partial positive character. Covalent character is shown by HCl as the atoms of hydrogen and chlorine share their electrons with each other.
What is HI polar or nonpolar?
Hydrogen iodide (HI) Notice the symmetry of the molecule: When divided, the top and bottom as well as the left and right are not mirror images of one another. One also knows the molecule is polar because the bond is polar.
Is HCl or hi more polar?
HCl is more polar than HI as the electronegativity difference between hydrogen and chlorine is greater when compared to the electronegativity difference between hydrogen and iodine.
Is HCl HF polar or nonpolar?
So, is HCl polar or Nonpolar? HCl (hydrochloric acid) is a polar molecule because the chlorine is more electronegative than hydrogen due to which it attracts the bonded electron pair slightly nearer to it and gains a partial negative charge and hydrogen gains a partial positive charge.
Why is HI more polar than HCl?
HCl is more polar than HI as the electronegativity difference between hydrogen and chlorine is greater when compared to the electronegativity difference between hydrogen and iodine.
Is HF a polar molecule?
The molecule HF is clearly very polar, meaning that a significant difference in electron density exists across the length of the molecule.
Which is strongest HCl HBr and HI?
Thus, order of acid strength is HI>HBr>HCl>HF.
Why HF is less polar than HBr?
c : Due to the higher electronegativity of F HF is more polar than HBr pure water contains H+ and OH– ions. In covalency sharing of electrons between two nonmetal atoms takes place.
Is HCl polar or nonpolar and why?
There and that the chlorine has the electrons. Much longer than the hydrogen.
Is HCl a polar or nonpolar?
polar covalent compound
HCl is polar covalent compound as the chloride ion is more electronegative than hydrogen ion. So chloride ion carries partial negative character while hydrogen carries partial positive character.
What type of polar is HF?
In a hydrogen fluoride (HF) molecule, a hydrogen atom and a fluorine atom are held together by a polar covalent bond.
Is HCl HBr or HI stronger acid?
Increasing order of acidity of hydrogen halides is HF<HCl<HBr<HI.
Why are HI and HBr more reactive than HCl?
Greater the nucleophilicity of the halide ion, more reactive is the halogen acid. Since the nucleophilicity of the halide ion follow the sequence I- > Br- > Cl- therefore , reactivity order of halogen acids with ethers is HI > HBr> HCl. Q.
Why is HCl is polar?
HCl is a polar molecule. This is because the Chlorine (Cl) atom in the HCl molecule is more electronegative and does not share the bonding electrons equally with Hydrogen (H). But H2 And Cl2 are non polar due to similar electronegativity of both the atoms in the molecule H2 And Cl2 .
How do you know if a molecule is polar?
Steps to Identify Polar Molecules
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory)
- Visualize or draw the geometry.
- Find the net dipole moment (you don't have to actually do calculations if you can visualize it)
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
